Your command center. Their companion app.
Configure trials in Participate. Engage patients through their phones. See results in real-time. This is how modern clinical trials maximize retention.
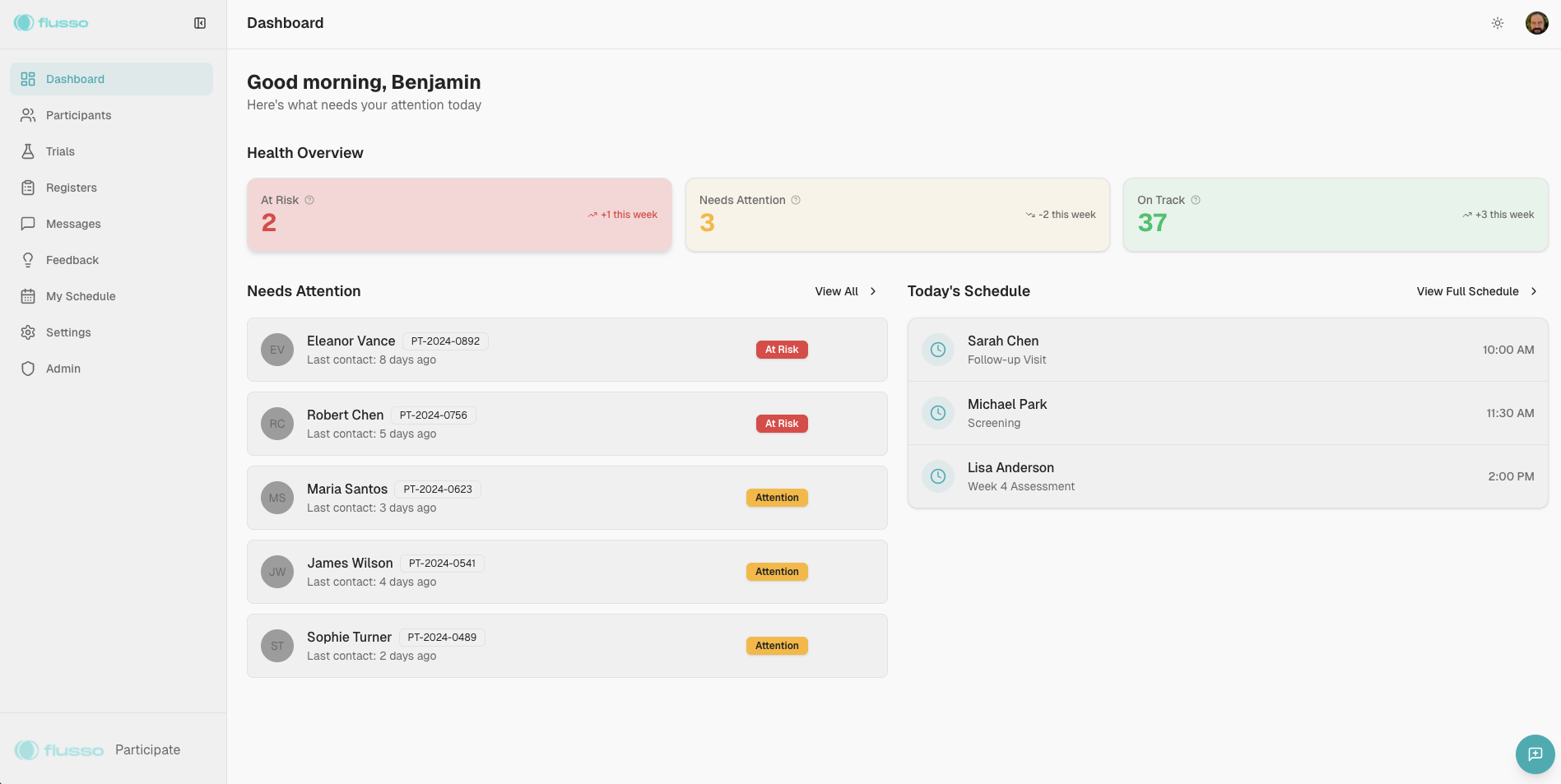
Clinical trials fail from both sides
Success requires solving problems for coordinators and participants alike
Clinic-Side Challenges
- Can't reach participants between visits—calls go unanswered, emails ignored
- Survey completion rates hovering below 60%
- No early warning system for dropout risk
- Manual reminder calls consuming coordinator time
- Fragmented systems with no unified participant view
Participant-Side Challenges
- Another portal to download? No thanks.
- Don't know what's expected of me or when
- Feel like a data point, not a person
- Forgot my login—again
- Information is scattered across emails, texts, and paper
You configure. They experience. You see everything.
Three core workflows that connect your trial management to participant engagement
Surveys & ePRO
Design your survey
Build ePRO questionnaires in Participate's visual builder. Set schedules, reminders, and completion windows.
One-tap completion
Participants receive a push notification, tap to open, and complete surveys in their familiar Engage app.
Real-time results
Watch completion rates live. Get alerts for missing responses. Export audit-ready data anytime.
Design your PRO surveys in Participate's intuitive builder. Participants receive a notification in Engage, complete the survey in under 3 minutes, and you see results instantly—no chasing, no phone calls, no missing data.
Messaging & Communication
Compose your message
Send broadcast updates to all participants or private messages to individuals. Schedule or send immediately.
Secure delivery
Messages arrive alongside their GP conversations—familiar, trusted, and always noticed.
Full audit trail
Read receipts, response tracking, and timestamped logs. Every communication documented for compliance.
Send protocol updates to all participants or private messages to individuals—directly from Participate. Participants receive them in the same app they use for all their healthcare, making your trial communication feel native, not intrusive. Every message is encrypted, timestamped, and audit-ready.
Visits & Scheduling
Build your schedule
Define visit windows, set reminder timing, and configure automated follow-ups for no-shows.
Smart reminders
Reminders adapt to each participant's preferences—time of day, frequency, and calendar sync.
Attendance tracking
See confirmations, cancellations, and reschedules in real-time. No phone tag required.
Define your visit schedule once in Participate. Engage sends intelligent reminders that learn participant preferences—some want 24 hours notice, others need a same-day ping. When participants confirm, cancel, or reschedule, you see it instantly.
Why participants actually use Engage
The secret to high adoption? Don't make them download another trial app.
Engage isn't just for your trial
It's their healthcare inbox. Participants already use Engage to message their GP, see their specialists, and manage their health records. Your trial becomes part of their daily routine—not another app they forget to open.
One app for all their healthcare
Participants already use Engage for their GP, specialists, and health records. Your trial becomes part of their routine—not another app to forget.
Trust built in
End-to-end encryption means participants trust Engage with their health data. That trust extends to your trial communications.
Designed for real people
Offline access, plain language explanations, accessibility-first design. Built by people who've been frustrated participants themselves.
Privacy they can see
Participants see exactly what data your trial accesses and why. Transparency builds the trust that keeps people enrolled.
The results that matter
Performance goals for research sites using our integrated platform
Metrics represent platform design goals based on industry benchmarks and projected improvements from integrated engagement.
*Industry benchmarks: Advarra/Tufts CSDD, PRO literature
One platform. Two experiences.
Everything connects seamlessly behind the scenes
Participate
Your command center
Engage
Their companion app
Participant's single healthcare app — alongside their GP, specialists, and health records
Built for regulatory reality
Compliance isn't an afterthought—it's embedded in every feature
HIPAA Compliant
Protected health information handled with appropriate administrative, physical, and technical safeguards.
21 CFR Part 11
Electronic records and signatures meet FDA requirements for clinical trial data integrity.
GDPR Ready
International data protection compliance for multi-region clinical trials.
ICH-GCP Aligned
Good Clinical Practice principles embedded throughout the platform design.
Complete Audit Trails
Every action timestamped and logged. Generate compliance documentation with one click.
See the difference integrated engagement makes
Join research institutions that are transforming their clinical trial operations with Flusso.
Talk to our team to see how Flusso can transform your operations.